The Problems with Hydrogen - Future Car
What is Hydrogen?
September 9, 2009
Hydrogen is the lightest element in the periodic table and the first listed. It is a colorless, tasteless, odorless, non-metalic gas that is highly flammable. Hydrogen can be ignited in an air (or oxygen) mixture from 4% of volume all the way up to 75% of volume. Hydrogen will spontaneously ignite at 932º F. I can also be ignited by spark, heat, or sunlight.
Hydrogen is thought to be the most abundant element in the universe.

Why Hydrogen as a Fuel?
Beyond the fact that hydrogen is so readily available by a wide variety of means, it is a very clean burning fuel. Yes, as it burns it produces a small quantity of Nitrous Oxides, but, by and large, its biggest "pollutant" is water. Pure water with few if any impurities.
As such it is a clear candidate as a replacement for hydrocarbon (gasoline & diesel) based fuels which produce carbon dioxide, carbon monoxide, nitrous oxide, and sulfur dioxide.
Also, because there are now two methods for utilizing hydrogen in automobiles (fuel cells and internal combustion engines) it is a far more attractive replacement than liquid fuels of any type.
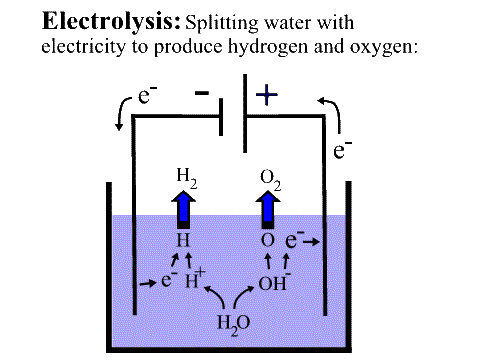
Converting Water to Hydrogen (and Oxygen)
There are many methods of converting water into hydrogen/oxygen. But most are inefficient, cost more in energy than is produced from the hydrogen, or cause the reactants or electrodes to degrade at such a high rate that parts replacement becomes economically nonviable. In short, no known method today, can produce a large quantity of hydrogen that is cheap to produce.
There are currently two hundred (200) different methods of producing hydrogen from water. Of those fourteen (14) are likely candidates for mass production if efficiencies can be improved.
- Zinc Zinc-Oxide Cycle: This process requires 3,452 °F (via solar towers) causing the zinc-oxide in the reaction to split into zinc and oxygen. This process is called thermal decomposition. The zinc is then allowed to cool to 800.6 °F and reacted with water. This produces hydrogen. This method has a 44% efficiency rating.
- Sulfer-Iodine Cycle: This process requires three separate steps. In it Iodide is reacted with sulfur and water and Hydrogen Iodide to produce Sulfuric Acid. The resulting water and sulfuric acid are separated by condensation. Iodide, water, and sulfur are then separated in a third step. The resulting gas is hydrogen. All chemicals can be recycled. High temperatures are required, however, and the corrosive properties of the chemicals presents a material challenge. Efficiency is about 38%
Electrolysis of water: This is a process of passing an electrical current through water. it is highly likely that every one of us have seen this process in science class. It is touted to have an efficiency of between 50% and 70%. Though this sounds better than the two methods above some of the current is converted to heat; a useless expenditure of energy. Also, this method does not account for a material problem with the platinum cathode and anodes. As the oxygen is produced at the anode and anodes are where oxidation normally occurs this electrode is subject to fast wear and rapid replacement.
At the time of this writing it is thought that hydrogen could be produced via electrolysis using wind-power as an energy source. In the near term a kilogram of hydrogen (roughly equivalent to a gallon of gasoline) could be produced for about $5.50 falling in price to $2.25 as the process begins to pay for itself.






Storing Hydrogen
Storing hydrogen at pump is really not a problem. Most proposals assume that the hydrogen will be produced on the site of the "gas" pump, thus alleviating storage problems. This would require building space for the equipment roughly equivalent to the size of a convenience store.
The real problem is storing hydrogen in the vehicle.
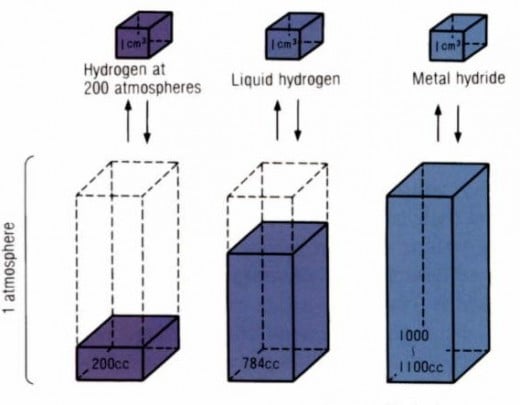
Gas: A gram of hydrogen at atmospheric pressure (14.7 PSI) occupies about two point nine (2.9) gallons of space (11 liters). To say that this is impracticable for a vehicle is a gross understatement. Putting hydrogen under pressure (say a minimum of 5,000 PSI) is a much more practical way to keep the hydrogen in a vehicle, but the cost in weight for the tanks and the room required for those tanks (about the size of an average car trunk) also makes this impractical . Then there's the engineering required of a tank capable of storing a gas at 5,000 ~ 10,000 PSI. As one might imagine a tank engineered to these specifications is quite expensive and they still take up more space than the average gasoline tank.
Liquid: Storing hydrogen as a liquid requires temperatures of minus two-hundred fifty-three (-253º C) degrees Celsius or minus four-hundred eighty-two degrees Fahrenheit (-482º F). The energy required to get the hydrogen to that temperature and maintain it in a storage tank in a car would far outweigh the energy extracted from the hydrogen when put to use. There would also be a problem with converting the liquid to a gas just prior to use, though this is minor compared to the storage problem.
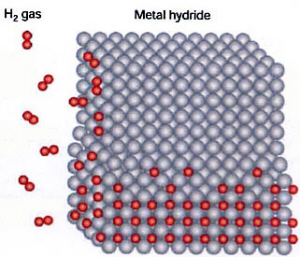
Metal Hydrides: Hydrogen can also be stored in a metallic matrix called metal hydride. A metal hydride is any metal that can bond with hydrogen without reacting to that hydrogen. (see graphic at right)
Stanford Ovshinsky, the father of the Nickle Metal Hydride Battery, currently has a patent on the storage of hydrogen in metal hydride matrix. The patent provides a means of storing hydrogen, under low pressure, in a metallic matrix. Ovonics, Stanford's company, calls the process "Ovonic Solid Hydrogen Storage."
Ovonics currently produces hydride canisters that hold eighty (80)*, twenty-five (25), nine (9), and six point seven (6.7) grams of hydrogen. These canisters are approved by the U.S. Department of Transportation. The largest of these canisters is 15" X 3.5" diameter. This is considerably less space than a gallon of hydrogen as gas at atmospheric pressure. Filled, the canisters weigh fifteen (15) pounds and store six hundred (600) watt equivalent of power. One drawback to this design is that it takes eight (8) hours at two-hundred fifty (250) PSI to recharge the largest canister. At this time I am unable to find a price for this canister.
There are other companies and inventors working on similar metal hydride storage system. I used the Ovonics example because it is one of the first and Stan Ovshinsky has a long and successful background with these materials.
* eighty grams of hydrogen is roughly equivalent to 8/100ths of a gallon of gasoline
Conclusion
It is obvious, to me at least, that we are not quite at a working solution for large scale hydrogen production, distribution, and refueling. However, advances in materials science, storage, and production of hydrogen tell me that the real solutions are mere years away.
In fact, they may already be here, only awaiting the inventor's patenting or funding process to reach the public.
This is the fifth in the series of the Future of the Car.
Future Car Series
- The Future of the Car
The car of tomorrow promises to be radically different than what we are driving today. Pardon the Science Fiction wording please.There are many reasons for these advances; Computing and programming A... - Future Car - Ford's Hydrogen I.C.E.
I.C.E. stands for Internal Combustion Engine. Ford is currently looking at creating a car that will bridge the gap between gasoline power and the very likely future fuel; hydrogen. The I.C.E. burns hydrogen... - Future Car - Chevrolet Volt
The Chevrolet Volt is a range extended electric that can be plugged into a standard wall outlet (120V). The vehicle to be produced by the General Motors Chevrolet division. It is expected to be launched as a... - Future Car - Chrysler EV Series
EV stands for Electric Vehicle. Much to everyone's surprise Chrysler recently unveiled an all electric vehicle called the Dodge Circuit EV. The Circuit will be an all electric sports car vaguely reminiscent... - Future Car - The Problems with Hydrogen
Hydrogen is the lightest element in the periodic table and the first listed. It is a colorless, tasteless, odorless, non-metalic gas that is highly flammable. Hydrogen can be ignited in an air (or oxygen)... - Future Car - Toyota / Honda
Sorry to join these two models in one hub, but neither company (Toyota or Honda) has a lot to say about the new models. Toyota in particular won't say anything about the upcoming Prius, but rumors abound. ... - Future Car - Subaru
Subaru is the western spelling for the Japanese word for - Future Car - Peugeot / Citroën
Peugeot and Citroën is now known as Peugeot Citroën. The companies are now combined though each is marketing and selling it's own particular models. If Citroën continues as a viable company remains to be... - Future Car - Opel
Opel, in coopreation with General Motors, has introduced the Ampera (or is it Flextreme?) as a diesel plug-in series hybrid concept car. It can travel thirty-four (34 mi) miles on its lithium-ion battery...